How To: Eliminate Off-Patent Competition & Defend Your Proprietary Drug
A guide for beleaguered Big Pharma executives.
Do you work for a large pharmaceutical conglomerate? Are you worried about repurposed off-patent drugs? Are you concerned that your brand new proprietary drug will have to deal with unfair competition by a molecule discovered 100 years ago? Have no fear, Do Your Own Research is here to help!
This all-in-one guide can help your quest to approve drugs you have monopoly rights over, while eliminating any competition from off-patent repurposed generics.
The secret is ultimately simple: leave no stone unturned. Optimize your chances every step of the way. Off-patent drugs have no deep-pocketed corporate sponsors (apparently they belong “to everyone”), so when it comes down to it, it’s like stealing candy from a baby. With this bulletproof step-by-step guide, your well-funded team of killer pros will be literally unstoppable!
Leave Nothing To Chance
The general who wins the battle makes many calculations in his temple before the battle is fought. The general who loses makes but few calculations beforehand.
- Sun Tzu
When designing your trial, you must make sure every last detail is fine-tuned to perfection. At the same time, you can rest assured the off-patent trials will not have the budget, incentives, experience, or determination to reach the same level of perfection. After all, the most talented and respected trial designers already work for you. Here are some useful talking points you can use with the press later:
Off-patent trial: "Administration delay doesn't really matter. Ten days from symptoms is good enough. It either works or it doesn’t, right?"
On-patent trial: "If the patient has had symptoms for more than three days, tough luck. Reject them immediately. Our drug is an antiviral and that means it only works early."
Off-patent trial: "You can’t possibly dose the drug above previously-proven safe doses. Serious scientists take established FDA package inserts to heart."
On-patent trial: "Nobody really knows how much is safe to give, and there is no FDA package insert, so go as high as you need to make it work, and we’ll deal with the adverse effects later. Better than dying of the virus, right?"
Off-patent trial: "Delivering the drug on time is expensive, use FedEx, the data should be useful anyway. It either works or it doesn’t, right?"
On-patent trial: "They will take the drug as soon as possible if we have to teleport the drug to their door, break into their house, and force it down their throats. We’ll be damned if we let grandpa mess up our trial."
Off-patent trial: "You can source the drug from one of many generic manufacturers of varying quality."
On-patent trial: "We have perfectly positioned every single atom in those pills in our high-end facilities. If we didn't feel moved to tears by a pill’s overwhelming perfection, we will throw it away and start over. No, we can’t do that when we mass manufacture, but that’s a post-approval problem, right?"
Off-patent trial: "Include almost anyone who is willing to be in your trial. Big trials are good for the investigators’ CV, and finding patients is expensive. Besides, data is data and you can’t afford to be picky. It’s an emergency!"
On-patent trial: "We will look far and wide for patients with the optimal profile for our drug. We will spare no expense to run the trial in multiple countries at once so we can get exactly as many perfect patients as we need. This obviously means it works for everyone."
Explain Away Positive Results
Despite your best efforts, sometimes the off-patent drug will fail to fail, while your proprietary Elixir of Life might not quite demonstrate the true extent of its magical powers of healing as strongly as you wanted. Have no fear, here’s how you can play it to your favor:
Say you have a trial with a weak signal:
Off-patent: "The trial demonstrated there’s no evidence it has any effect whatsoever. The Science has spoken! Time to stop all further research!"
On-patent: "We see a positive trend with significant improvement in specific high-risk subgroups. We will start giving it for compassionate use while running a more finely-tuned followup study."
Off-patent trial: "You did an exploratory trial on very few patients, so you did not reach significance. Therefore this is evidence your drug failed."
On-patent trial: "We only run massive trials so that even the slightest difference can be clearly visible."
Off-patent drug: "There is no individual trial demonstrating statistically significant evidence of reducing mortality when used as early treatment."
On-patent drug: "If you squint, it seems to have improved a surrogate of a surrogate endpoint. Approval of our life-saving compound is certain!"
Off-patent drug: "The trial showed a huge effect. We're not sure why, so it must be fraud. It's not plausible a drug works this well."
On-patent drug: "Our drug works so miraculously well we stopped the trial early. Who needs surprises, amirite? Now, could you please give us an EUA, prepay a few billion dollars so we can manufacture it, and bribe hospitals to use it? kthxbai!"

If all else fails, go for the jugular:
Off-patent trial: "The positive result is probably because of the other thing the drug is famous for being effective for."
On-patent trial: "Nobody has ever used this thing for anything so even if it works for some contingent reason, you'd never know it."
Defend Negative Results
Just as important as explaining away any positive results, you must exaggerate, promote, and provide support for any negative results. Here’s how you can do that:
Off-patent drug: "Nah, it shouldn't matter that some people in the control group were probably taking it anyway. It's randomized, right?"
On-patent drug: "Nobody even knows what our drug is, never mind having access to it or being able to even come near it outside a trial setting. Good luck finding PF-07321332 in your local pharmacy.”
It doesn’t matter if it was early, late, they gave too little, or gave too much. If the result is out there, you can use it to your advantage:

Deploy Fear, Uncertainty, and Doubt
When all else fails, just make sure they are confused and fearful. Most people don’t have time to dig into the details, and by the time they’ve done that, it’s too late.
Off-patent drug: "Some trial used an obviously irresponsible dose, poisoning its patients, therefore the drug isn't safe and can’t be used."
On-patent drug: "Only make it available to handpicked investigators who would never dream of dosing at anything other than your perfectly designed regimen."
Off-patent drug: "Perfect safety in pregnancy and reproduction has not been proven therefore it’s not really safe, is it?"
On-patent drug: "Wear a condom for at least 30 days after taking your last pill. Just in case. Oh, no real reason, why do you ask? Yeah, we started testing in pregnant women six months ago. I mean, sure, they’ve not given birth yet, but why is that relevant? People are dying!!!"

If authorities are on your side, cite them. If not, ignore them:

Off-patent drug: "Mechanism of action is unclear, so the results are probably fraud."
On-patent drug: "Mechanism of action is unclear, but that's true for so many other approved drugs. Isn't the human body wonderful?"
Go After The Researchers
When push comes to shove, you may have to go after the annoying doctors and scientists that keep producing positive trials for the off-patent repurposed alternatives. Given that money is clearly not their objective, they’re probably weird in other ways too. Maybe they’ve even pissed off other people in the past. Dig up any dirt you can and make sure the press knows, too. And if you can’t find anything, well, use your imagination:
Off-patent trial: "Investigator is conflicted: they said something nice about the drug at some point and didn’t declare a conflict in the paper—ignore trial and/or meta-analysis."
On-patent trial: "So what if the investigator receives millions of dollars from the pharmaceutical that makes the drug? This is how science works! How dare you!"
Off-patent drug, negative meta-analysis: “So what if the lead author was caught on video confessing that his sponsor dictated the conclusion? He was just being pragmatic, and anyway, we made sure he looked like he was right in the end, so really, he’s actually a hero. What’s a little academic misconduct if you’re saving the world?”
Off-patent trial: "Paper is not written in perfect English, and some tables have typos. Obviously, the investigators must be incompetent. Ignore trial."
On-patent trial: "We employ experienced medical writers who are specialized in producing flawless manuscripts ready to be signed off by the investigator who will put their name on them. And if a flaw or a dozen still make it to the paper, well, that’s what minor corrections are for. Science is a self-correcting process, right?"

Off-patent trial: "The investigator has been implicated in some other bad trial at some point. Out of an abundance of caution, we have to ignore this one too. We can’t take chances. People’s lives are at stake!"
On-patent trial: "Look, yes, the manufacturer has paid the most in fines for fraud of any corporation in history, and funds the largest government lobbies the world has ever seen, but it’s not fair to hold that against this drug. People’s lives are at stake!"
Off-patent drug: "Someone got excited and called it a miracle drug once. This is a violation of scientific neutrality standards and we can't take anything they ever say again seriously. Make sure this is mentioned every time the drug comes up."
On-patent drug: "Even one life saved is a miracle of science and we have a moral obligation to shout it from the rooftops. Anyone who expresses doubt is a traitor to all that is good in the world."
Off-patent drug: "No respectable scientist advocates for it. By which we mean that if anyone advocates for it we will drag their name through the mud and try to get their medical license taken away. See? Not respectable anymore!”
On-patent drug: "If you don't advocate for it you're not respectable. Are you trying to kill people?"

Make Meta-Analyses Sound Unconvincing
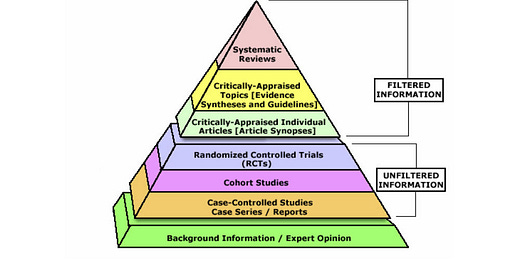
Despite your best efforts, sooner or later somebody is bound to do a meta-analysis or systematic review, taking the many small trials and pooling their evidence together. This may sound like an insurmountable problem, since this kind of review is literally at the top of the evidence-based medicine pyramid:
But what’s better than one systematic review or meta-analysis? Many systematic reviews or meta-analyses! By the time they figure out which is which, your drug is approved and the regulator will say there’s no need for another one. Here are some tips:
Off-patent drug, positive meta-analysis: "Some trials in your massive meta-analysis were fraudulent, therefore the meta-analysis must be thrown out. I don’t care if you can easily recompute it, science demands perfection.”
Off-patent drug, negative meta-analysis: “When we throw out almost all the evidence, and balkanize what’s left so it can’t be composed, ok, look, we still see a statistically significant result, but hey, wouldn’t it be nice to wait for some more trials? Science can’t be rushed!”
On-patent drug: "Meta-analysis? Lol. We only have one perfect huge trial and nobody can even touch our drug before approval.”
Meta-analyses will often cover a longer period, during which time variants may need different dosing.
On-patent drug: "Boy, who could have predicted variants, huh? Anyway, take more."
Off-patent drug: "That trial was under a different variant so any positive results are invalid." (You can keep the negative results, those are fine.)
Invest In Post-Publication Takedowns
Off-patent trial: "Not sharing data is a red flag and you're probably a fraudster."
On-patent trial: "Oh, we could not possibly share our proprietary data. And what about patient confidentiality? Yes, I know we promised to share, but you'll have to take our word for it."
Off-patent trial(2): “Oh good, you shared your data voluntarily online. Let us punish you by running a bunch of unknown tests and coming up with a complicated argument to say some minor fault should get your study retracted. If you don’t like it, sort it out with the journal.”
Off-patent drug, positive trial: "The endpoint was changed from the protocol. That’s probably fraud. This is why we pre-register, you fool."
Off-patent drug, negative trial: "OK, look, we chose a completely different endpoint than we said we would, but we had a very good reason for it. No, you can't see the data for the original endpoint."
Off-patent drug: "Just accept the negative trials from our highly respected colleagues. It just doesn't work. Stop complaining about wanting the perfect trial, it either works or it doesn’t, right?"
On-patent drug: "We have fine-tuned every single aspect of this trial. We will make sure it doesn't look like it’s not working for any reason we can possibly prevent, and if for some reason the truth of its efficacy is not self-evident and we can’t spin it to seem like it is, we’ll make sure the data gets out as late as possible, on a Friday night after a terrorist attack, or something."
Use The Regulator and the Press To Your Advantage
The final call is in the hands of the regulator and the court of public opinion. Lucky for you, you are set up for success:
Off-patent drug: "FDA won't consider it because there's nobody there to push them through the process. The last thing a bureaucrat wants is more work. Encourage the press to write whatever they want, politicizing the issue. Who’s going to go after them anyway?"
On-patent drug: "FDA is funded by your pharma dollars. And you will make sure to pay top-tier people to make sure all the forms are perfect. Hire the best PR people to ensure the press behaves well. What’s a high-end dinner or an invite to the Met Gala when billions of dollars are at stake?"
In Conclusion
I know this sounds like a lot of work, but ultimately it’s just money. Easy money that you will earn back in multiples, to be precise. After all, even though most of these tricks are well-known and many have written about them, people have short memories:
In Case of Emergency, Remember, There’s Always the C-Word. Deploy it Like So:
"Hey guys, are we sure we should trust the people that have been messing with the evidence for decades without checking their work?"
"CONSPIRACY THEORIST!!!"
"Hey guys, I think these doctors from all over the world are somehow producing these consistent results to get us to eat horse dewormer."
"YEAH, THAT MAKES PERFECT SENSE!!!"
With all these tricks up your sleeve, you may tear society to pieces, and you may destroy trust in the medical establishment for generations. But what matters is one thing and one thing only: Your. Drug. Will. Get. Approved.
After all, if you didn’t do it, somebody else would have been in your incredibly luxurious, incredibly unaffordable, tailor-made-in-Italy-just-for-you, shoes. What good is sentimentality if you’re broke? Besides, you don’t want to be like the poors who can only afford off-patent generics when they get sick, do you?





I pictured Shakespearean characters reading each part. Love this
Would you rather take horse medicine or medicine no animal should take?