This article is part of a series on the TOGETHER trial. More articles from this series here.
Disclaimer: this article will be heavy on calculations, though it does come with a juicy payoff. If you don’t have the mental bandwidth to walk through it, feel free to skip it, and wait it out until my next post on this which (hopefully) will be one that summarizes the findings so far, without doing the heavy lifting of showing my work.
Consilient Sleuthing
A guiding principle in my analysis of the TOGETHER trial is that the large number of issues seen are not distinct, but clues to a root issue with the execution of the trial. A previous article presented my hypothesis as to what the root anomaly was, but I’m still connecting loose ends. One of the main mysteries remaining unresolved is the size of the 3-day placebo subgroup, as well as some strange properties it seems to have.
Who’s In the 3-dose Placebo Subgroup?
A huge point of confusion has been the fact that the placebo arm in the TOGETHER trial was split into subgroups taking different placebo regimens. So, for example, some placebo patients would be on a 3-day regimen, identical to what the high-dose ivermectin group was taking. Some others would be on a 10-day, twice-a-day regimen, mimicking the fluvoxamine arm. Towards the end of the period of interest, 1-day and 14-day placebo subgroups were added, increasing confusion further.
The first question we need to answer is: how many people were in each placebo subgroup? The TOGETHER ivermectin paper tells us that the number of people with 100% compliance (took all 3 doses) in the 3-day placebo subgroup were 288.
Let's try to derive this number on our own, based on what we know from the enrollment chart and the other publications. The first question we need to ask is about the way in which the patients were split into groups. A reasonable assumption—for which I have some degree of private confirmation—is that patients were allocated to placebo regimens at a proportion equivalent to the number of active arms at any given time. So if three treatment arms were running at a certain time, and one of them was using 3-dose regimen, then 1/3 of placebo patients enrolled during that time would get the 3-day placebo.
Applying this allocation approach to the numbers we've extracted from the enrollment chart, something incredible shows up: the number of patients that would be enrolled in the 3-day placebo arm appear to be exactly 288.
The table is straightforward, except for the first week of 3-day placebo patients, where only 1/4 of the allocation to the protocol is made, since the switchover to the new protocol and allocation algorithm appears to have happened on the latter part of that week and affected exactly a quarter of the patients in the group.
The data for this table—up to week 64—has been extracted from the enrollment chart shared by the principal investigator on August 6th, containing obviously truncated data over the last few weeks, presumably as the information was still being gathered. The fluvoxamine preprint gives us enough information to backfill those numbers, and the final paper on fluvoxamine gives us the data to complete the last week of the period of interest.
There may be some small rounding errors at the boundaries, but whatever they are, they appear to be canceling each other out, because we seem to have derived exactly the number that the authors report.
If true, this would unearth two new major issues with the study:
1. This would imply that all 288 patients had 100% adherence to the 3-day protocol, whereas it is clear that of the 679 high-dose ivermectin patients, only 624 did. I can think of no honorable way to explain the absence of drop-off in the 3-day placebo group, which theoretically had the same amount of information as the treatment group.
2. To get to 288, we need to include placebo patients from March 4th onwards. This is really concerning because it implies that either the patients started to be allocated to 3-day placebo from early March—even though the protocol that indicated such a split placebo approach was not approved by the local Brazilian ethics board (CONEP) until March 15th (or according to the authors on March 21st)—or the paper's authors have retroactively categorized patients who did not receive a 3-day placebo as having received it. Both of these possibilities have grave implications for the conduct of the study.
Placebo Patient Mortality
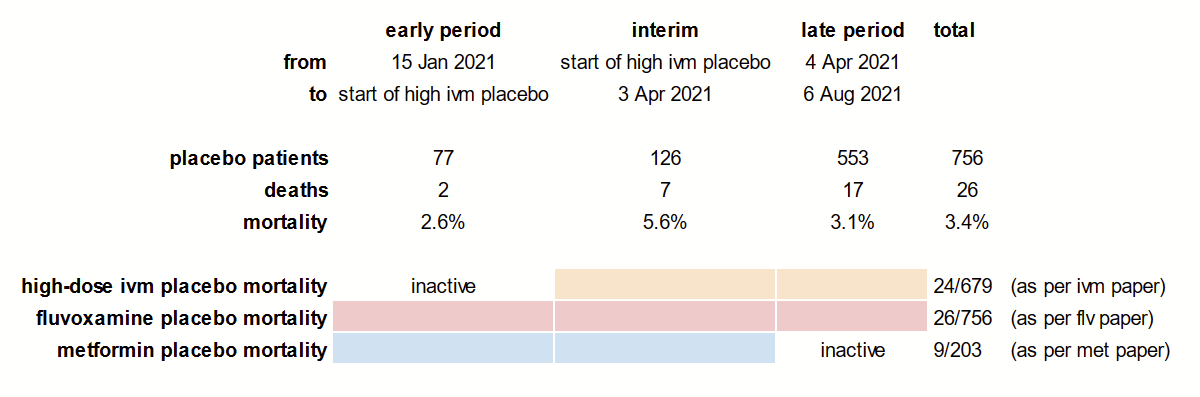
We can derive additional information on the study’s background mortality by using the data we have on placebo patients. My previous article included the following diagram, but perhaps a little more explanation is in order.
To explain the figure, let’s start from the list of clues we have from the series of publications related to the trial:
- The metformin paper, covering the period between Jan 15-Apr 3, 2021 had 9/203 mortality in the placebo group.
- The fluvoxamine paper, covering the period between Jan 20-Aug 5, 2021 had 26/756 mortality in the placebo group.
- The ivermectin paper, covering the period between March 4-Aug 6, 2021 had 24/679 mortality in the placebo group.
The authors of the ivermectin paper state that the placebo group consists of patients recruited from March 23rd onwards, but I've demonstrated in a prior article the reason why this must be, charitably, an error. Regardless, the precise data we define here should not make a big difference for this analysis, and if anything, taking the authors at their word makes the conclusions of this analysis worse. Regardless, we will use the approximate March 4th date for now, which is in the vicinity of the only sensible answer.
The previous articles in this series also discuss why the fluvoxamine placebo patient set is almost certainly the complete placebo patient set for all three studies, despite the small misalignment in dates. To avoid restating the analysis, I will proceed with this as given.
With calendar matters behind us, we can start extracting a bit more information from our data:
Subtracting the ivermectin placebo statistics from the fluvoxamine placebo statistics, we get a useful look at the early placebo patients:
Between Jan 20th and Mar 4th, there were (756 - 679 = 77) placebo patients enrolled, among which (26 - 24 = 2) died (2.6% mortality).
We can do the same for the period between April 3rd and August 5th by subtracting metformin placebo from fluvoxamine placebo patients:
Between Apr 3rd and Aug 5th, there were (756 - 203 = 553) placebo patients enrolled, among which (26 - 9 = 17) died (3.1% mortality).
By subtracting this last period from the ivermectin placebo patients, we can find out something about the peak of the Gamma variant among the placebo patients of TOGETHER:
Between Mar 4th and Apr 3rd there were (679 - 553 = 126) placebo patients enrolled, among which (24 - 17 = 7) died (5.6% mortality).
This month-long period between March 4th and April 3rd seems to have spectacularly high mortality. And if we use what we know from the overall CFR in the area at the time, mortality must have been concentrated to the latter half of this period, after patient enrollment for the high-dose ivermectin arm started.
What's the Mortality of the 3-day Placebo Subgroup?
The authors have not given us this number, but knowing when patients were enrolled into the 3-day placebo subgroup, and knowing the mortality from the analysis in the first section, we can make some pretty interesting observations:
[Update: I discovered a significant error in my calculations while reviewing this post and the following section is materially revised, closer to the conclusion I expected when originally writing this article. Apologies, will triple check my calculations in the future]
First, in my model, the 3-day placebo group shows mortality of 3.43%, whereas the full placebo group has 3.53%. That already shows there’s an asymmetry between the two groups, but perhaps there will be differing opinions about the importance of the asymmetry.
However, these calculations are assuming that mortality stays the same between March 4th and April 3rd. What we showed with our CFR calculations in the previous section is that indeed there is a vast difference between the early and late part of that period. What would happen if we assume that mortality stays the same as in the previous period, around 2.6%, until March 23, and then we increase mortality in the next period, ending April 3rd, enough so that the overall mortality rate of the placebo group stays about the same?
Fascinatingly, you can see that placebo patient mortality both for 3-day and in general stays about the same. However, what shoots up is the last column. That column tracks what the deaths in the treatment group would be like if the treatment did nothing. Mortality goes from 3.51% in the prior table, roughly similar to the placebo group, to 4.21% in this second table. To make the difference clearer, the expected deaths for the high-dose ivermectin treatment arm increase by about 4.5 deaths.
The study showed a difference of 3 deaths between treatment and placebo (21 vs 24 deaths). In our modeling exercise, we find that if we make a high but plausible hypothesis of mortality in the two critical weeks between March 22nd and April 4th, the expected mortality of the treatment group jumps from ~24 to ~28.5. This is 150% of the difference observed, inflicted as a penalty to the treatment arm.
Is this the actual difference in reality? We don’t really know, as we don’t have the data. Even if it is 20% of that, it’s a significant confounder in the study.
As always, please feel to type up your comments and (especially!) corrections in the comments. More than anything else, my objective is to get this right, especially given the gravity of the concerns that are emerging.
This article is part of a series on the TOGETHER trial. More articles from this series here.







Are you implying what we are thinking, that the reason the IVM placebo arm did so well, better than the other placebo groups, is because they were unblinded due to IVM being available over the counter in Brazil, so the IVM placebo patients realized they weren’t getting IVM and just went and bought their own?
I love your work Alexandros but my question is where is it going from here?
The “fraud busters” would probably have taken everything you’ve done so far to the media and declare they’ve discovered fraud, which would then make major news.
I’m guessing you are going to be a bit more circumspect than that and approach the journal with your concerns or write a letter to the editor with questions for the authors.
Is that the plan?