Andrew Hill, in his paper titled “Ivermectin for COVID-19: Addressing Potential Bias and Medical Fraud“, concludes that clinical trials must urgently up their game:
With cases of potential medical fraud now identified, it is essential that access to patient-level databases be provided. If authors fail to provide these data, the study should be considered with a higher index of suspicion. Additionally, it should be mandatory that all registered trials report their findings. We understand that these are substantial changes to established procedures. However, the failure to recognize the potentially fraudulent studies, which led to multiple meta-analyses suggesting significant benefits of ivermectin for COVID-19, indicates that the tools currently used to evaluate the quality of clinical trials are insufficient. These events warrant our stringent recommendations.
Taking his warnings seriously, I thought perhaps the best place to start would be the winner of the “Trial of the Year,” as awarded by the Society for Clinical Trials. I’m talking about none other than the TOGETHER Trial:
After all, if the “Trial of the Year” isn’t meeting these transparency guarantees, what chance do we have demanding them from anyone else?
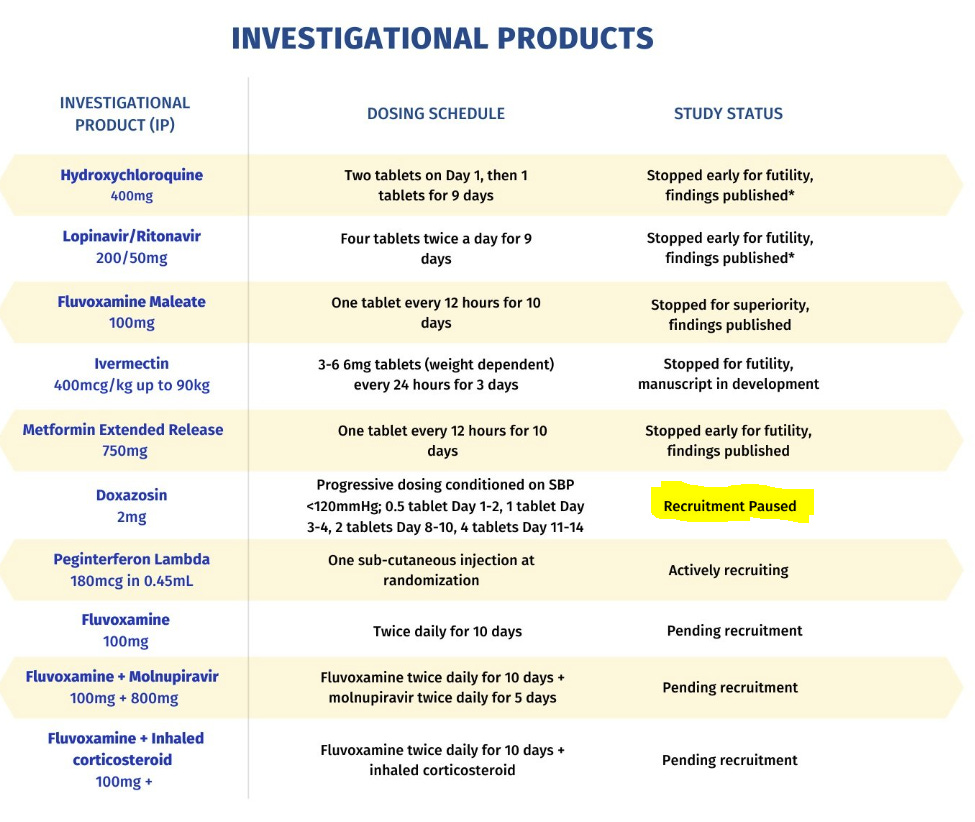
As it turns out—in materials released by the trial in 2022—there are some arms for which we have not seen any data released by the trial authors. The IVM (low-dose) arm (or single-dose, as I prefer to call it), is described as ending in March 2021. Similarly, the Doxazosin arm is noted as having ended in September 2021. If you squint, you can see all this in the illustration below:
So what happened to the data in these two arms? We are really not sure, but there are some clues we can gather from the study materials. Let’s dive in:
What Happened to Single-Dose Ivermectin?
The single-dose ivermectin arm was the original ivermectin arm of the trial. It started recruitment some time in January 2021. While we don’t know the exact date the first patient was recruited, according to a presentation released by the trial, it appears to have been sometime in the week of January 25th, 2021.
By February 15th, a new protocol had been drafted and was submitted to the Brazilian authorities on February 19th. It recommended substituting the single-dose ivermectin arm with a three-dose arm. Despite the fact they claimed to believe the new dosing would deliver the “best clinical results,” they continued to recruit patients into the single-dose arm, which, presumably, they did not think would deliver the best clinical results.
As we’ve previously discussed, the low-dose ivermectin arm was paused some point in early March 2021. I estimate that day was March 4th or 5th. The TOGETHER trial Twitter account says it happened definitively by March 9th:

No official results have been released, but if the rumors on the street are true, it showed single-dose ivermectin performed worse than placebo, which would be evidence of a dose-response relationship, given that the three-dose ivermectin arm—even with its capped dosing and other trial issues—showed an 80% probability of superiority over placebo. There are other factors that could explain such a relationship, so it would be excellent if the results were indeed released, so they can be evaluated.
What Happened to Doxazosin?
The trial website says that Doxazosin is in “recruitment paused” status.
The trial’s protocol version history mentions this in version 3.1, which was dated November 16, 2021:
Closed Doxazosin treatment arm to enrollment.
Trigger was met at a planned interim analysis.
As of August 5th, the fluvoxamine paper in the Lancet mentions that there had been 91 patients allocated to doxazosin and 96 to peginterferon-lambda.
The numbers disseminated with the ivermectin paper are different, reporting that 121 patients were allocated to doxazosin, and 170 to peginterferon-lambda. This is very likely a snapshot from the September 12th, 2021 mini-interim analysis.
From the Brazil protocol v5.0, dated November 20, 2021, we learn that:
On the occasion of the 4ª interim analysis that took place on August 5, 2021 and after blinded data analysis recommended:
(…)
(5) The conduct of a specific interim analysis to evaluate adverse events associated with the doxasozine arm, since there will be a projection of at least 125 patients included in the doxasozine arm (considering 1:1 randomization), to be scheduled by September 15.
(…)
On September 12, 2012, the 5th interim analysis was conducted specifically to assess adverse events regarding the doxasozin arm, and the trial's independent data and safety review committee recommended discontinuation of the 14-day treatment arms.
Indeed, the clinicaltrials.gov record for the TOGETHER trial indicates that it was dropped from the protocol in the January 2022 revision:
As a result, we can conclude that after about 120-125 patients recruited, or thereabouts, the DSMC concluded that the adverse event profile of doxazosin was not worth whatever benefit was hypothesized, and decided to end the arm.
Did 200 Patients Participate for Nothing?
Between the 77 patients recruited into the single-dose ivermectin arm, and the 120-125 patients recruited into the doxazosin arm, we have approximately 200 patients that were recruited, randomized, treated, monitored, and had their results collected and analyzed. But the results of these arms have not, to my knowledge, been shared with the broader scientific community. Assuming low-dose ivermectin did not make a difference—as my information suggests—and that doxazosin was interrupted due to its safety profile at the dose being tried, this is important information that can help researchers set the dosing of future trials.
The authors don’t even have to put this up for publication at a major journal, or even write a full-blown preprint. A simple preliminary report with accurate numbers will do just as well. Is that not worth it, in the name of setting a good example for other scientists across the world?
At the very least, they owe it to the patients who participated in the TOGETHER clinical trial in the hopes of contributing something to humanity.







Why did Ed Mills partner with Pontifica Universidade Catholica de Minas Gerais (World ranking #1401) rather than Universidade Federal de Minas Gerais (World ranking #456)? Both are in the same city, Belo Horizonte.
Universidade Federal de Minas Gerias is the largest Federal university in Brazil and ranked variously as #5 or #9 in Latin America. Pontifica Universidade Catholica de Minas Gerias does not have an individual ranking but rather is ranked in the zone #101-125 for Latin American universities.
The PUC universities in Brazil are privately funded and very expensive, at least compared to the Federal universities where tuition is minimal for those applicants who have passed the "vestibular," an exam like SAT.
I would like to see Mills justify his choice of university partner, but if he won't release basic data, then mine is a forlorn hope.
Thank you for your careful detailed analysis. I hope it prompts answers.