This article is part of a public peer review of Scott Alexander’s essay on ivermectin. You can find an index containing all the articles in this series here.
I’d like to thank Dr. Avi Bitterman for reading a draft of this and providing feedback. While we don’t necessarily agree on everything I write here, discussing this topic with him has made this a much stronger writeup, so I’m grateful for the time he devoted to talking it through with me.
The Strongyloides Worms Hypothesis
Briefly, the hypothesis in question is that prevalence of Strongyloides stercoralis worms in a population alters the results of ivermectin studies done in that population. The results make ivermectin seem effective for treating COVID-19 in high Strongyloides prevalence areas, when in fact, it is not—at least not directly.
There are at least two ways that Strongyloides could interfere with the results of ivermectin studies:
Ivermectin doesn’t help much against COVID-19, but people not taking ivermectin are dying more because patients hospitalized with COVID-19 are commonly given steroids. These suppress the immune system and cause previously suppressed worms to multiply aggressively; a process called hyperinfection. This is especially dangerous when patients are denied ivermectin due to medical oversight.
Ivermectin helps COVID-19 patients by eliminating worms infections, therefore preventing deaths that way.
The Short Answer
If you’re short on time, my answer to the title question is that I’m not convinced. My rationale, which this article will expand on, is as follows:
If either branch of the hypothesis was true, we would expect to see control groups in high-prevalence studies with substantially higher death rates than control groups in low-prevalence studies, since either prior worms infection or untreated hyperinfection would make it more likely for control group patients to die. This has not been shown. If anything, the opposite seems to be the case.
A reasonable response may be to assert that there are other differences between the groups of countries and regions with high-prevalence that may mask the effect. However, those other shared factors may also be invoked to explain the difference in the effectiveness of ivermectin. So unless a clear case is made to explain the discrepancy, the hypothesis is, at the very least, incomplete.
In the paper published to support this hypothesis, when Strongyloides prevalence estimates are adjusted so that the studies can be properly compared, the observed correlation becomes much weaker.
Regardless of the strength of the association—this being a correlational study—there are many factors that could explain any difference between groups. As such, we need to investigate the individual studies for signs that this specific confounder played a role.
Specifically for deaths in the control group due to hyperinfection, when investigating the high-prevalence studies, I found that the large majority of the deaths were very unlikely to have been caused by hyperinfection. While I can’t rule out that one or two of the 18 deaths were caused by hyperinfection, the evidence cuts against the hypothesis that sufficient number of deaths were due to hyperinfection to meaningfully alter the results of the ivermectin studies:
The assumption that doctors across the world would have missed a substantial fraction of deaths being due to hyperinfection is unlikely. While this could happen in some cases, for the hyperinfection hypothesis to have a meaningful effect, it would require hyperinfection to be a relevant factor in 10% of deaths in high prevalence areas or more, which is inconsistent with the totality of evidence.
The Long Answer
Let’s walk through the evidence and expand on the reasons for my position:
The JAMA Meta-Analysis
Scott Alexander’s article relied on an analysis that JAMA recently published—a paper by Bitterman et al.—that makes the case for the Strongyloides hypothesis. You’d have to read the paper to get the full argument, but briefly, it says that if we take the studies with mortality as an endpoint from the Bryant et al. meta-analysis, and separate them by the prevalence of Strongyloides in the relevant countries or regions, we see a strong tendency for the more positive studies to be associated with high-prevalence.
Treatment of worm infections might reduce the negative effect of COVID-19! And ivermectin is a deworming drug! You can see where this is going…
The most relevant species of worm here is the roundworm Strongyloides stercoralis. Among the commonest treatments for COVID-19 is corticosteroids, a type of immunosuppresant drug. The types of immune responses it suppresses do more harm than good in coronavirus, so turning them off limits collateral damage and makes patients better on net. But these are also the types of immune responses that control Strongyloides. If you turn them off even very briefly, the worms multiply out of control, you get what’s called “Strongyloides hyperinfection”, and pretty often you die.
…
Dr. Avi Bitterman carries the hypothesis to the finish line:
This hypothesis is not the sort of thing you casually ignore. There are enough features about it that make it intriguing and worth investigating. From reading the JAMA paper, it really seems like there might be something that requires explanation.
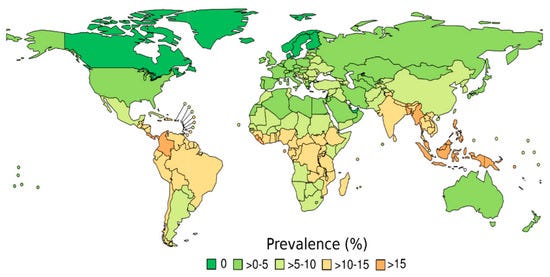
The first issue raised—by those who were not convinced by the paper—is the apparent incongruity in the data sources used for Strongyloides prevalence. You see, for nine of the studies, the authors used the Buonfrate et al. study, whose Strongyloides prevalence map looks something like this:
Notice how Brazil is in the >10-15% zone, which would be squarely within the “high-prevalence” zone.
Specifically for Brazil, however, the authors used data from a different study, Paula et al., which describes its conclusions thus:
The objective of this review was to outline an epidemiological profile of Strongyloides stercoralis by parasitological and serological diagnosis in inhabitants, and to associate this profile with different immunosupression situations, in Brazil, over 20 years (1990–2009). The occurrence of S. stercoralis using parasitological methods was 5·5%, being 4·8% in rural and 5·0% in urban areas, characterizing the country as hyperendemic.
Here’s where things get strange: one study, Buonfrate et al., places Brazil at a prevalence of 11.2%, with a 95% confidence interval of (5.7% - 16.6%). This is significantly above the 8.1% global prevalence computed in the same paper, which acts as a cutoff between the high- and low-prevalence groups. The other study, Paula et al., claims that Brazil has a 5.5% prevalence. That is not just low, it’s so low it’s outside the 95% confidence interval of Buonfrate et al.! Stranger still, based on their result, Paula et al. characterize Brazil as “hyperendemic.”
What’s going on? Well, it appears that the two papers used different methods to compute their results, which means we can’t really compare the two results. It’s like comparing prices in US dollars and Canadian dollars without first adjusting for the exchange rate.
I should give credit to Fred Stalder (aka. @sudokuvariante) for first raising this issue:
Specifically, while Paula et al. used parasitological methods (i.e. stool sample-based) to compute their result, Buonfrate et al. used an adjusted set of both parasitological and serological methods (i.e. blood sample-based), meaning that the results cannot be directly compared. The paper in JAMA says:
For the variable of strongyloidiasis prevalence, country-level prevalence by parasitological methods and more granular within-country regional prevalence estimates where possible were used
Given that Buonfrate et al.—which is used for nine of the eleven studies compared—uses a mixture of parasitological and serological methods that have been adjusted so that they can be combined, we have to consider the blending of adjusted and unadjusted numbers a straightforward oversight, but perhaps one that we can rectify post-hoc.
Instead of trying to find the perfect way to adjust the measurements, or reverting purely to the numbers in Buonfrate et al., I decided to split the difference and try to keep the regional Paula et al. data in the mix for Brazil.
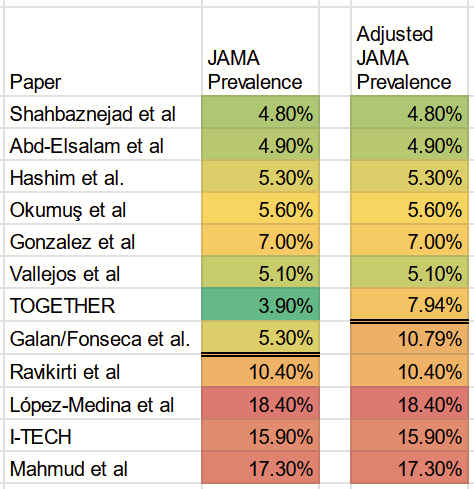
If we take the 5.5% Brazil prevalence according to Paula et al., and compare it with the 11.2% Brazil prevalence from Buonfrate, we can calculate a multiplier of 2.04. This is our “exchange rate.” The JAMA paper attributes to one of the Brazilian studies—Galan/Fonseca et al.—a prevalence of 5.3%, while the other Brazilian study— TOGETHER—was assigned a local prevalence of 3.8%. This is data sourced from the Paula et al. study.
By using the multiplier, we can compute adjusted prevalence rates of 7.94% for TOGETHER and 10.79% for Galan/Fonseca et al.
Please note that Galan/Fonseca et al. is not Szente Fonseca et al. as is referenced in the JAMA paper. (I’m told this was an error inserted in the editorial process by the journal itself). The correct reference is to the paper ivmmeta calls Galan et al. Tess Laurie’s group calls it Fonseca et al., so Galan/Fonseca et al. is the identifier we’ll use in this article. Hopefully, we can keep it all straight.
Here are the updated prevalence numbers: published numbers to the left; numbers after my adjustment to the right:
If we apply the 8.1% cutoff used in the JAMA paper, this implies we’d need to move the Galan/Fonseca et al. trial to the high-prevalence group.
I asked Dr. Bitterman—first author of the JAMA paper—what his analysis would look like if we made that adjustment. To his credit, he sent over the modified result.
I want to take a moment to praise that move because he had no reason to do that. This adjustment shows that the published result changes substantially if we reclassify Fonseca. An author who was committed to defending his paper at all costs would—upon seeing the adjusted result—find some excuse or some other reason to obscure that result. Dr. Bitterman showed no signs of wanting to do that. That level of honesty is pretty rare in these highly polarized times, and I want to give credit where it is due.
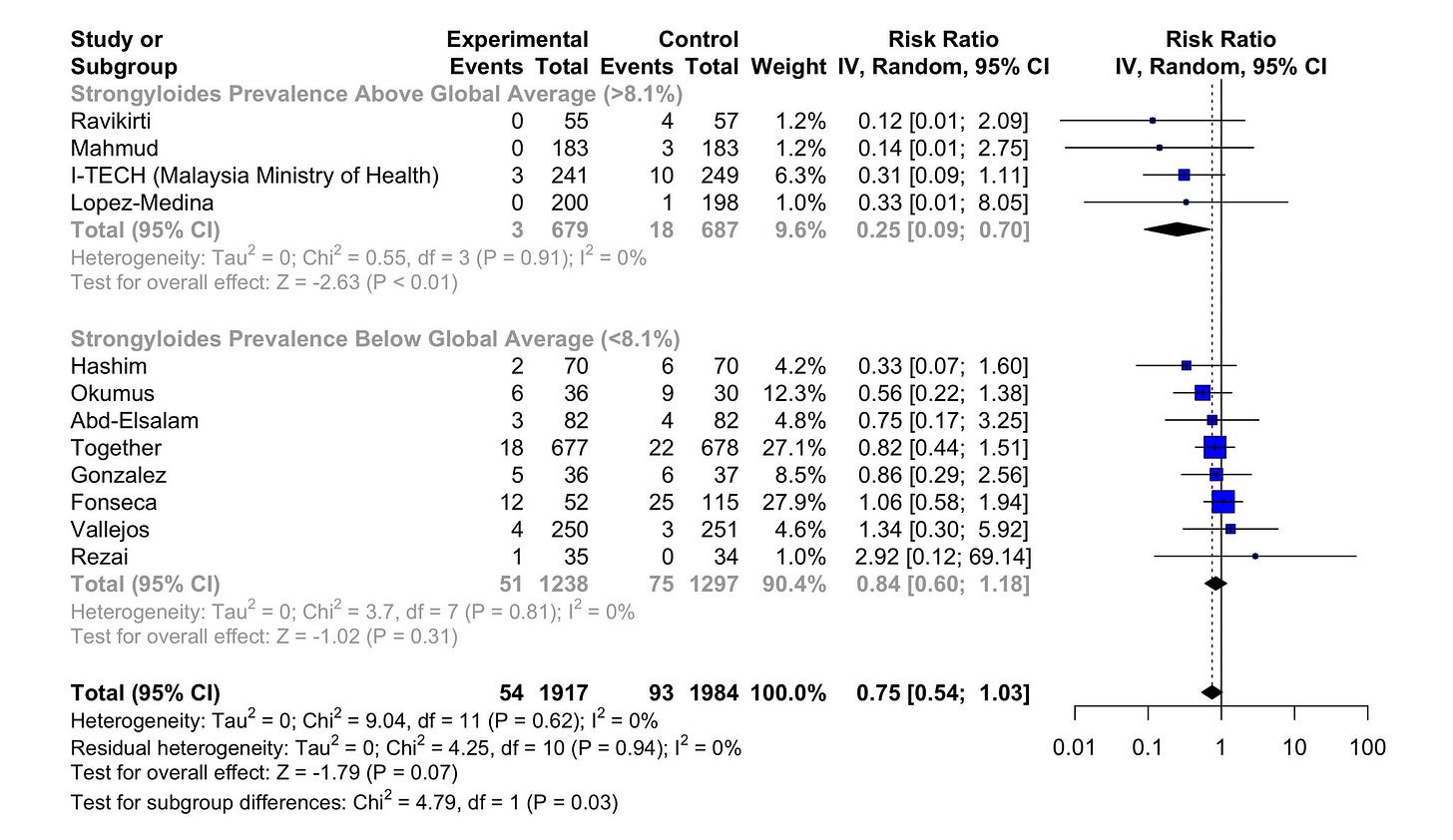
Here are the results of the updated analysis. These are not the exact results Dr. Bitterman sent me—as I wanted to replicate the published paper a bit more closely—but they are not meaningfully different:
In this reanalysis, we see that the statistical significance for the modified high-prevalence group is eliminated, even though the point estimate still shows some advantage. In technical terms, the test for subgroup differences goes from p=0.03 to p=0.35. Since I am going by Bayesian standards, this doesn’t necessarily mean that there is “no difference” in the two results, but it is much more likely that such a result could emerge by chance than the one that was published.
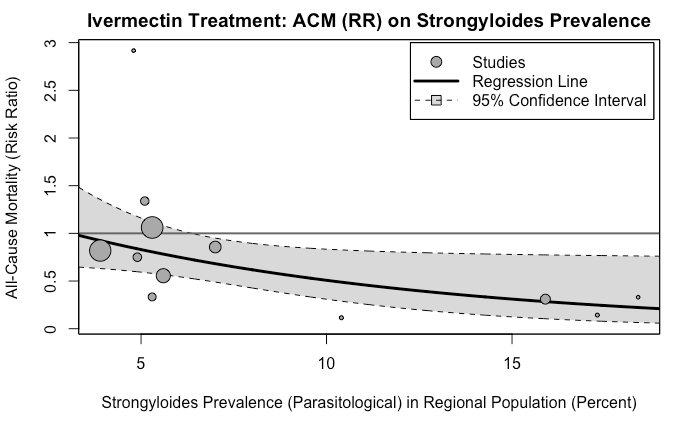
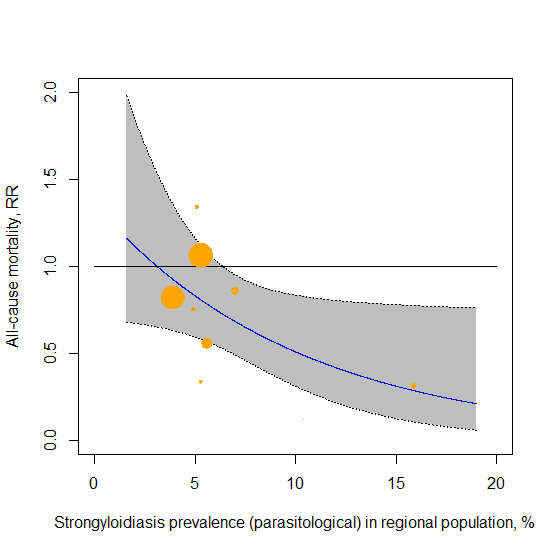
Dr. Bitterman also mentioned that the more important result, in his view, is the meta-regression showing the correlation. Indeed, that is a method that is free of the issue of how many subgroups to make and where to draw the line, so I concur it is a superior approach to answering the question. Here is the diagram from the published paper:
It would indeed be very interesting to see what happens if we use the adjusted prevalence score above, which would move the two largest bubbles towards the center of the range.
However, I lack the statistical capabilities to run such an analysis. As such, I’ll have to leave it at this, unless a capable reader cares to supply it. (If you’re up for working on it, my Twitter DMs are open.)
<UPDATE>: Many thanks to pure mathematician Dr. Daniel Victor Tausk, for making my dreams come true. First, he replicated the analysis of the original JAMA paper:
Then, he re-ran the meta-regression with the adjusted prevalence rates:
Here’s how he described the analysis he did and what the results were (emphasis mine):
The metaregression is based on a model of the form:
log(risk ratio) = a.prevalence + b + random error terms;
the coefficient a expresses the size of the association between worms prevalence and efficacy of ivermectin (since efficacy = 1 - risk ratio, a positive association corresponds to a negative value of a). The p-value for the test a=0 expresses how much evidence we have for some association between efficacy of ivermectin and worms prevalence.
In the JAMA paper they estimated a = −0.0983 with p-value = 0.046 for the test of a=0. I was able to reproduce these numbers exactly (as well as the entire forest plot in the JAMA paper and the recalculated forest plot from your article).
If I rerun the metaregression replacing the JAMA prevalence with your adjusted numbers I get:
a = -0.0517, with p-value = 0.3413 for the test of a=0.
So, the original effect size was reduced and it went from barely significant to not significant at all.
</UPDATE>
One thing that is beyond doubt, is that once we adjust the prevalence rates, the subgroup correlation significantly weakens. Instead of debating how much weaker that correlation is, we can take the reasonable next step and explore the ivermectin studies most likely to have suffered deaths by hyperinfection to see if their data shows any signs of such an event happening.
As the title text of the famous XKCD strip reminds us, correlation may not imply causation, but it does say there’s something worth investigating qualitatively.
To do that, however, we must first build up some context.
How Fast Does Hyperinfection Kill?
When I first saw the Strongyloides hypothesis, I wondered if deaths by hyperinfection versus deaths by COVID-19 could not be distinguished by symptoms, timing, or otherwise. In order to find that out, we need to first understand the speed at which hyperinfection can kill.
In questions such as this one—where the data is sparse on the ground and no definitive answers exist—we must combine multiple sources of information to make our best estimation of a result. In particular, we have three different signals to work with:
Historic Case Review
The first and most obvious place to look is the largest set of hyperinfection in the literature, a paper descriptively named Strongyloides stercoralis hyperinfection syndrome: a case series and a review of the literature.
The authors gathered 133 studies by examining the literature and also finding more unpublished cases from France (emphasis mine):
111 (83.5 %) patients were treated with corticosteroids at a median dose of 40 mg per day. Of note, the corticosteroid dosing had been recently increased prior to [Strongyloides Hyperinfection Syndrome] in 34 patients (46.6 % of available data). The median time from corticosteroid treatment initiation to the occurrence of [Strongyloides Hyperinfection Syndrome] symptoms was 42 days
What they found is that the median number of days from initiation of corticosteroids to identification of Strongyloides infection was 42 days, with 75% taking more than 14 days, and 25% taking more than 90 days. Unfortunately, Dr. Bitterman highlighted that there is some ambiguity here. In one place in the paper, the authors describe this number as “Time from initiation [of steroid treatment] to the occurrence of SHS symptoms,” which is not the same endpoint.
Thankfully, this ambiguity does not subtract from the overall takeaway, which is that hyperinfection takes several weeks to kill. Could this be because of treatment of the patients with e.g. ivermectin? Or perhaps due to the fact that faster cases are not spotted?
For that, we turn to a different, and more controlled experiment.
The Marmosets
There’s a fascinating paper called Strongyloides stercoralis infection in marmosets: replication of complicated and uncomplicated human disease and parasite biology, which infected marmosets with Strongyloides larvae, then gave three of the animals high doses of dexamethasone, and then observed what happened.
Though we may have our reservations about using these cute little primates for these experiments, the researchers make a good case for why these animals were chosen:
The inability to maintain S. stercoralis of human origin in the laboratory and the nearly complete absence of viable models for the study of auto-infection, hyperinfection and dissemination of this parasite have hampered our understanding of various aspects related to the biology and pathophysiology of this parasite. Marmosets are susceptible to infections with a number of parasites of medical importance [64]-[70], and the use of these animals has advantages over other non-human primates: smaller size (i.e., weight of only approximately 400 g), greater abundance of some species in nature, ease of captive breeding and handling, reduced maintenance costs and a relatively lower risk for the transmission of zoonoses to humans [56],[64],[71],[72].
The results are pretty much what we’d expect: all three marmosets (P2, P6, P8) died, though it did take quite a few weeks for them to die:
P2: given dexamethasone 29-35 days after infection, died 57-63 days after infection, so 21 days (min) to 35 days (max) from steroid administration to death.
P6: given dexamethasone 22-28 days after infection, died 71-77 days after infection, so 42 days (min) to 56 days (max) from steroid administration to death.
P8: given dexamethasone 22-28 days after infection, died 106-112 days after infection, so 77 days (min) to 91 days from steroid administration to death.
Were the deaths due to the high dose of steroids? It doesn’t seem so:
The animals inoculated with dexamethasone exhibited reversible adverse signs related to treatment with glucocorticoids, such as oedema and skin changes, indicating immunosuppression during and after the administration of the drug. However, the main changes in the natural course of infection were observed later, culminating in the typical scenario of hyperinfection followed by dissemination of S. stercoralis and death of the host.
We also learn that…
The marmosets that received dexamethasone exhibited more severe clinical manifestations due to hyperinfection and dissemination of the parasites. Prior to the deaths of these animals, in addition to intestinal manifestations, the late involvement of the respiratory system was observed. In particular, in marmoset P2, the nervous system was also affected.
These marmosets were not treated with ivermectin or any other antiparasitic, since the objective was to examine the course of hyperinfection. So the length of time it took to die cannot be explained by the treatment. It confirms our suspicion that hyperinfection and/or dissemination—whether affecting humans or other primates—takes weeks or even months to kill.
COVID-19-Era Case Review
To make absolutely certain I’m not missing something, I put together my own list of COVID-19-related cases of steroid-triggered Strongyloidiasis that I could pull from the literature. To my knowledge, this is the largest list of its kind. Two papers that attempt to gather these have six and eight cases respectively, so this is substantially larger. Two more people have looked through the literature and not found more cases, and neither could the readers of this Substack.

In these cases, we found that only 5/13 of the cases were hyperinfections, and of those, only two lead to the death of the patient. In both cases, the patients were discharged from the hospital and later readmitted with hyperinfection symptoms. While it’s important to note that these percentages should not be seen as representative, perhaps there are some things we can calibrate on this basis. In one case, we only know it took more than 28 days from the initiation of steroids—but not clear how long—and in the other case, we know it took 53 days for the patient to die.
In this dataset, as in the others, it appears that Strongyloides hyperinfection takes several weeks to kill.
So, How Fast Does Hyperinfection kill?
Given that all three lines of evidence point to hyperinfection taking several weeks to kill the host—in the vast majority of cases—this is the answer we’ll have to go with. It doesn’t mean that some portion of cases don’t develop a lot faster, just that it isn’t common. Each line of evidence has significant objections that could be raised, but when we put them together, I see no way to avoid the obvious conclusion that hyperinfection canonically takes many weeks or even months to kill. I’m interested in evidence to the contrary, so please let me know if you come across anything by leaving a comment.
Qualitative Approach: Investigating Control Group Deaths in High-Prevalence Studies
With this background in mind, let’s dive into the four studies in high-prevalence countries that show the outsized effect according to the JAMA paper. We’ll explore how plausible hyperinfection is as an explanation for those deaths. In the paper, four studies are shown as being in high-prevalence areas, so we’ll focus on deaths in their control groups.
For each study, I’ve examined:
Strongyloides prevalence in the area
Steroid administration frequency and timing (where available)
Control group symptoms
Time from steroids administration to death
Individual patient data review (where possible)
Any other relevant factors
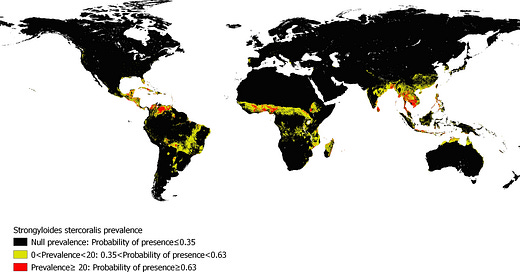
While most of the data comes from the studies themselves, for prevalence data we can look at the high-resolution map of Strongyloides stercoralis probability of presence that Krolewiecki’s team produced:
Without further ado, let’s dive right in with our first study!
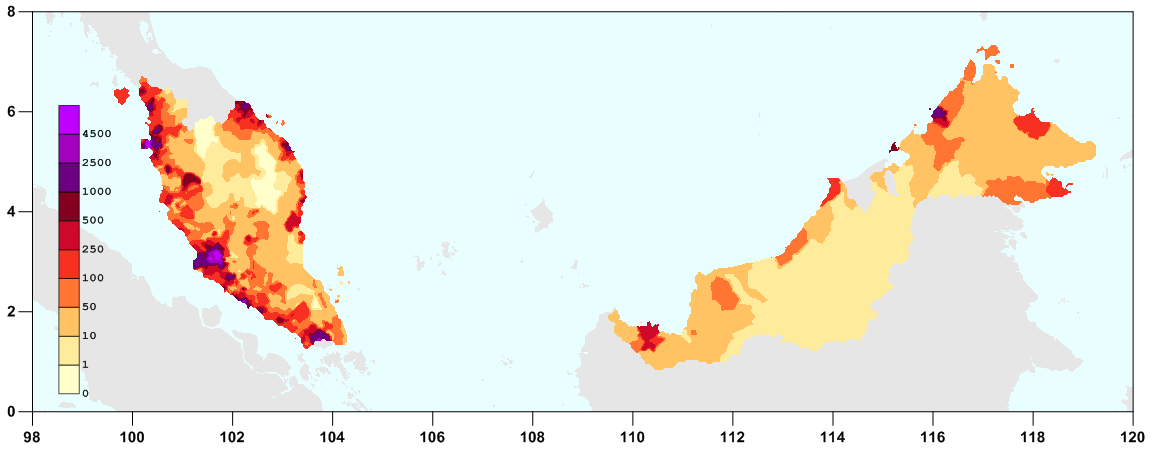
Study 1: I-TECH (Malaysia)
Strongyloides Prevalence
This study recruited patients in 21 different sites and hospitals across Malaysia:
Trying to infer prevalence of Strongyloides in the study’s patients is an interesting challenge, since we’d have to know how many patients were enrolled at each site. Seeing as most of the sites were on the south/east coast of the continental portion of Malaysia, eyeballing says we should expect moderate presence of Strongyloides infections, with strong variation between sites:
This is especially true when we consider the probability of prevalence in conjunction with Malaysia’s population density:
Steroid Administration
Corticosteroids were administered to 26.5% of the control group during the study period, while six patients had received corticosteroid treatment in the seven day period before joining the study.
Not 100% like some other trials, but certainly not a negligible amount, either.
Control Group Symptoms
The study reports zero cases of nausea, constipation, and abdominal pain in the control group, which are some of the hallmark symptoms of hyperinfection. There are some cases of diarrhea, but those are not out of line with what would be expected for COVID-19.
Dr. Bitterman highlighted that four or five of the deaths in this study were due to or involved sepsis. However, sepsis is common as a way to die from COVID-19—and for mechanically ventilated patients, unfortunately its occurrence is all too common. In fact, a paper reviewing the evidence concludes that:
“…the severe forms of COVID-19 should be considered as viral sepsis” and also notes that, “As expected, ventilator-associated pneumonia (VAP) (389, 50%), bloodstream infections (183, 34%), and catheter-associated bloodstream infections (CABSI) (74, 10%) were the most frequent [healthcare-associated infections].
Time From Steroids Administration to Death
This study reported ten deaths in the control group, and three deaths in the treatment group. These deaths are measured as 28-day mortality. Steroids would have to have been initiated very early, and the death would have occurred towards the upper end of the 28-day range to give enough time for hyperinfection to do real damage. For example, if steroids were given on day three, that leaves 25 days for hyperinfection to develop to the point of killing the patient. This is on the low end time wise, according to the case series. For that to explain the seven excess deaths in the control group, it would have to be quite the coincidence, though we can’t rule it out.
Individual Patient Data Review
I’ve not been able to access individual patient data so far.
Any Other Relevant Factors
One more hint can be seen by reading through the study authors’ responses to comments posted on the JAMA website. One in particular is worth reading in detail:
Here, we see that when a patient was ventilated, the treating physician decided to withhold the 5th dose of ivermectin. This is clear evidence that the hospital doctors can and will violate the study protocol when they believe the patient’s life is in danger. It’s reasonable to assume that—at least for this study—if the treating physicians detected that a control group patient was suffering from hyperinfection— and the correct course of action was to administer ivermectin—they would do exactly that, study protocol be damned.
Evaluation
At a stretch, I could see how one or even two of the ten deaths in the control group of I-TECH could be due to hyperinfection. To believe that most of the deaths were from hyperinfection, I would need more evidence.
Evidence That Would Meaningfully Shift My Conclusions
If it were demonstrated that the cases of diarrhea in the control group were disproportionately concentrated in the patients that ended up dying, I’d definitely get curious. Similarly, if it turned out that most control patients who died did so on the upper end of the 28-day observation period and were given steroids very early, you’d have my attention. The prior for either of those is low, which is what would make it noteworthy if either of these propositions were true.
Study 2: Lopez-Medina (Colombia)
Strongyloides Prevalence
Colombia is another high-prevalence country. Even though Strongyloides is not as present on the western side of the country, the city of Cali, where the study took place, does seem to be near a minor hotspot, so we should pay attention:
Time From Steroids Administration to Death
This trial had a single patient—part of the control group—who died between day 11 and day 15, according to the study appendix. If they were started on steroids—say on day 8—that leaves 3-7 days for them to develop hyperinfection and to die of it. Even if steroids were started earlier, this is exceedingly fast for the cases of hyperinfection we’ve seen in the literature.
Individual Patient Data Review
After I wrote the above, I recalled that someone I knew had been provided raw data by Dr. Lopez-Medina himself, so I could, in fact, find out whether the patient who’d died had been given steroids. The answer—according to the raw data of the Lopez-Medina study—is “no.” While for other patients, it is indicated that they were given prednisone, dexamethasone, or methylprednisolone, this particular patient was given antibiotics, but not steroids. Similarly, the patient does not appear to have been taking steroids for other reasons. It is unfortunate to learn that a medication that we now know can make a big difference for patients was not given in this case.
Evaluation
We can conclude with a high degree of confidence that in this case, steroid-triggered hyperinfection was not the cause of death.
Evidence That Would Meaningfully Shift My Conclusions
Hard to imagine what that might be—short of an autopsy of the patient who died—alongside a misplaced prescription for steroids.
Study 3: Mahmud (Bangladesh)
Strongyloides Prevalence
Without a doubt, this was a study in a high-prevalence area. You can see on the map above that Bangladesh is colored red, and it’s for a reason.
Steroid Administration
No mention of corticosteroids in the medications given to the patients, though they were part of the Bangladeshi treatment guidelines at the time, so there’s a chance they were given steroids. (Though if they were, why would they not be mentioned?)
Individual Patient Data Review
Thankfully, Mahmud and team have posted their dataset online, which I converted to CSV with PSPP. Looking at the data, it’s easy to see that the three patients who died had no incidence of diarrhea—which is mild evidence against the hypothesis that they died of hyperinfection.
[Pre-publication update: Dr. Bitterman—seeing the same data—also noted that two of the three patients that died had vomiting noted as a symptom which, correspondingly, is mild evidence in favor of the hypothesis that those two patients died of hyperinfection.]
We also learn from inspecting the records that one patient died in 12 days of illness, nowhere near enough time to die of hyperinfection. The other two died within 30 and 32 days of illness. If we assume steroids were given on day five, we have patients who had 25 and 27 days after initiation of steroids to develop—and die of— hyperinfection.
Evaluation
Once again, the time to make a Strongyloides hyperinfection death likely is on the lower bound on what would be required, making it unlikely.
Evidence That Would Meaningfully Shift My Conclusions
If we get evidence that steroids were given early during the patients’ treatment, that will certainly shift the probabilities in favor of hyperinfection.
Study 4: Kirti (India)
Strongyloides Prevalence
India is a big country, so perhaps the specific location can tell us more about the likelihood of Strongyloides infection in the local patients. As Scott Alexander describes it, we should expect similar probabilities as Bangladesh:
Here we’re in Eastern India - not exactly Bangladesh again, but a stone’s throw away from it.
Well, I sure hope he can throw a stone 218 miles (351 kilometers), because that’s the straight-line distance between the AIIMS hospital in Patna, Bihar, India, to the border of Bangladesh, as the crow flies (if said crow flew straight east for some reason):
So what happens if we try to localize Patna on the Krolewiecki Strongyloides probability of presence map? We see that in the vicinity of that city, Strongyloides is either very rare or non-existent (at least using this ecological model), and nothing like the very high-probability region of Bangladesh where Mahmud took place. This is a very rough geolocation, but it should be good enough as a sanity check.
As luck would have it, there is also a pre-pandemic study that investigated 838 patients from the city of Patna. These patients were suspected of intestinal parasites, so stool samples were taken and examined. Of those, 51 were found to have some kind of worm infection, but none of them had Strongyloides:
Steroid Administration
The trial gave 100% of its patients steroids—apparently—though they don’t say which and how much.
Control Group Symptoms
The paper has no mention of symptoms, making it hard to infer if there were any hyperinfection deaths.
Time From Steroids Administration to Death
This is a trial with four deaths in the control group and none in treatment arm. What we do know, is that the trial tracked patients until discharge from the hospital, after which point it seems to completely lose track of them (see the large number of PCR tests). Both of the deaths we saw earlier—that were linked to COVID-19 and corticosteroid administration—involve patients being discharged from the hospital for their original COVID-19 diagnosis, then coming back later with hyperinfection symptoms. This trial seems like it would not have followed those patients after discharge, and therefore not recorded those deaths in its endpoint.
Evaluation
I conclude that given the evidence of low local prevalence, and the likelihood that followup was short, it’s highly unlikely that Strongyloides played a big role in the control group patient deaths.
Evidence That Would Meaningfully Shift My Conclusions
More information about followup time, symptoms, as well as other kinds of individual patient data may shift the probabilities somewhat, as would any information that implies more than minimal local presence of Strongyloides.
Study 5: Galan/Fonseca et al. (North Brazil)
Strongyloides Prevalence
Another study that is worth looking into is Galan/Fonseca et al., which classifies as high-prevalence after our adjustment. When we geolocate it on the Krolewiecki map, it definitely shows up as a study worth digging into, as it’s fairly close to a regional hotspot:

Steroid Administration
From the paper:
Almost all participants in the three groups were treated with corticosteroids, most commonly, dexamethasone and methylprednisolone […]
Well, that certainly simplifies things.
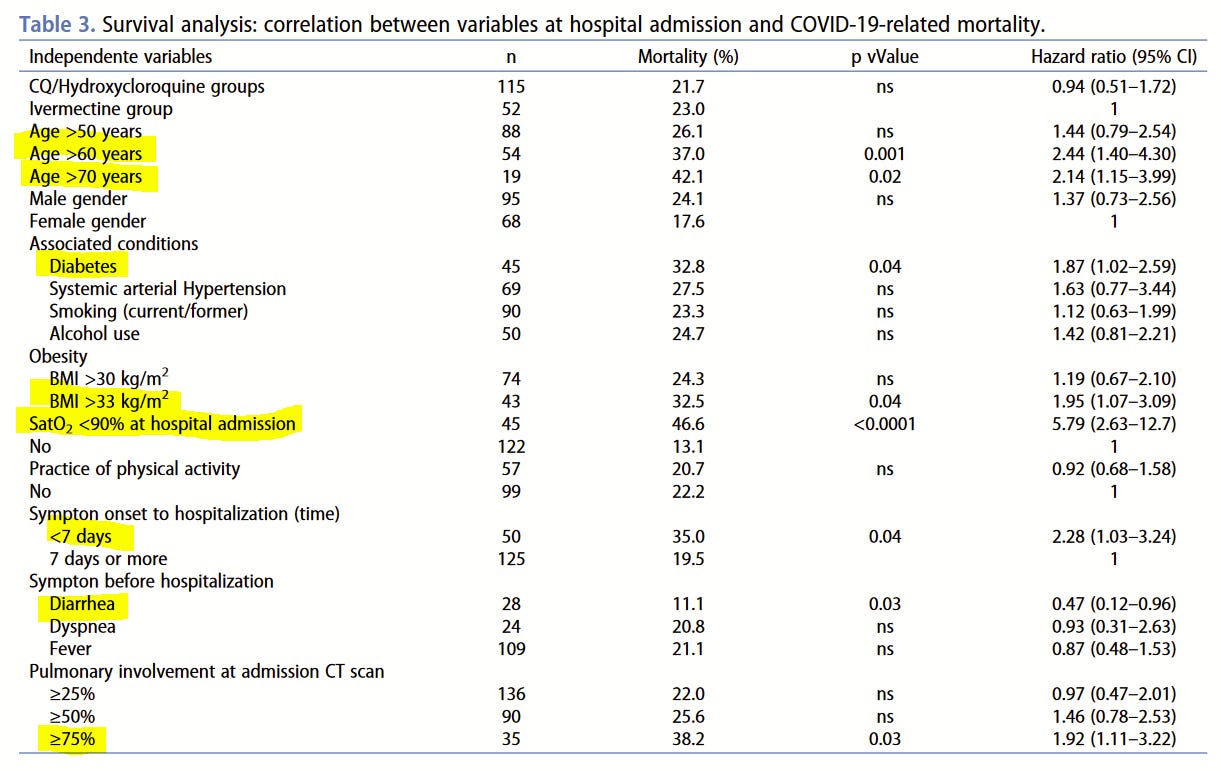
Control Group Symptoms
The paper gives us a good survival analysis from which we learn that mortality was associated with all the sorts of things you might expect for COVID-19 deaths: being old, obese, having low blood oxygen levels at admission. These factors make it individually more likely to die of COVID, though on the other hand, having diarrhea made it much less likely to die. All this cuts against the hyperinfection hypothesis in these particular cases.
Time From Steroids Administration to Death
In this study, we see a very similar number of deaths in both groups. The ivermectin group has 23% mortality, while the comparator group has 21.7%. Note that I didn’t call it a control group—the patients not given ivermectin were given HCQ or CQ instead—making it very difficult to interpret the results of the study, since there was no actual control group per se. However, luckily, the paper does include a Kaplan-Meier curve for the three groups—IVM, HCQ, CQ—from which we can get substantial information about the mortality in the study. Here’s the figure, which I’ve lightly modified with guides to make it easier to see some important numbers:
The first thing we see clearly is that patients start to die pretty fast upon hospitalization—and die steadily until day 18—at which point 19% of the patients had died. By comparison, only 2-4% more patients died over the remaining 72 days of observation. This leaves very little time between the potential initiation of steroids and the time of death for most patients.
Evaluation
Despite the indications of non-trivial local prevalence of Strongyloides, the Kaplan-Meier curves make it highly unlikely that the deaths we see were due to Strongyloides hyperinfection syndrome.
Evidence That Would Meaningfully Shift My Conclusions
If there’s any interaction between CQ/HCQ and Strongyloides I missed, that might explain the apparent speed of death for most patients. I would definitely give this another look.
Qualitative Review Conclusion
Looking at these five studies individually, I fail to see a strong signal that hyperinfection played an important role in any of the control group deaths. These studies are very different, as are the contexts within which they were executed. Some seem to have more “yellow flags” than others, and while we cannot rule hyperinfection out conclusively for each death, the data are inconsistent with what we would expect to see if Strongyloides hyperinfection was driving a substantial increase in deaths of control group patients.
Odds of Misdiagnosis
To believe the Strongyloides hypothesis, we’d expect one of the doctors in all five countries discussed above to have seen one or more deaths by hyperinfection—and even more cases that didn’t lead to death. Yet there is no mention of such an occurrence anywhere in their papers or otherwise.
Now, there’s always the chance that the doctors did not notice it. By the way, hyperinfection—at least in subset of the cases, can look like this—pretty hard to miss:

You can search Google Images for “Strongyloides hyperinfection” if you have the stomach for it. There is no shortage of material.
If indeed not one, but many doctors across the world made the same error, then we’d see some of the same phenomena in far greater numbers of non-trial contexts where steroids were given for COVID-19. However, the dearth of case reports of hyperinfection leading to death in the context of COVID-19 and steroids is hard to square with that set of assumptions. As far as I can see, there are only two cases of COVID-19-linked and steroid-triggered hyperinfection death in the literature, one of them from the USA. While case reports should not be taken as a representative sample, if we had even 10% of deaths in the relevant regions due to hyperinfection, we’d see more than the minimal chatter we observe now.
In Conclusion: What Do We Have to Believe?
In the startup/VC world, when looking at speculative investment cases, we often phrase the decision in terms of “What do you have to believe to think this is a good investment.” So here’s my analysis of the worms hypothesis using a similar rubric:
If the results are due to worm elimination helping with immune system function:
We’d need to ignore the fact that overall mortality for the control groups in high-prevalence studies is lower, not substantially higher, as we’d expect if worms were making prognosis worse.
If some other factor, like severity of the cases, makes the particular difference less of a problem, it also introduces a different variable we need to look into as explanatory for any correlation.
Even if true, this would in effect constitute a location-dependent mechanism of action—not necessarily a way to explain away the results. People convinced by this argument should insist ivermectin be used for treatment anywhere Strongyloides exposure may be suspected.
If the results are due to hyperinfection:
Patients in these trials died unusually fast for hyperinfection, and left no obvious trace of their particular mode of death in study data.
Many doctors across the world messed up in a big but very similar way, as they did not notice that patients suffered from hyperinfection—even though these countries are specifically high-prevalence countries where doctors would be aware of the risk—and despite visible signs of hyperinfection on occasion.
Until some of these patients are shown to have died of hyperinfection—and we have evidence that hyperinfection kills a lot faster than we have been led to believe by the literature—I find it extremely hard to be convinced by the thesis that worms explain the positive results we’ve seen for ivermectin. It’s an interesting hypothesis, and I can’t rule out that it acted as an effect modifier—perhaps one or two of the 18 deaths in the relevant set of trials might have in fact been due to hyperinfection—but the picture we see when we dive in is not consistent with the strong form of the hypothesis—that the majority of the deaths were in fact Strongyloides hyperinfections.
This article is part of a public peer review of Scott Alexander’s essay on ivermectin. You can find an index containing all the articles in this series here.


















Of all the articles you have written I think this one is the most transferable to a formal scientific paper.
You have obviously spent an incredible amount of time on this and I think with not much extra work you could truncate this analysis into a publishable paper.
Seriously consider doing this and specifically I would recommend submitting it to the JAMA.
You could maybe even consider offering Scott Alexander and or G M-K to co author. Additionally, if and when you are ready to submit I would very carefully document the process. If nothing else this might make for a good addition to Phil Harper’s Research Cartel documentary if the paper is (more than likely) rejected
In the link below, Dr. Pierre Kory reviews the suppression of ivermectin research by "prestigious" medical journals. Might be of interest to readers of Alexandros's peer review of S. A.'s post on ivermectin research.
https://pierrekory.substack.com/p/the-criminal-censorship-of-ivermectins?utm_source=substack&utm_medium=email